MPX1-OPV1-RPP30DNA
3-plex assay to simultaneously detect monkeypox specifically in the channel, Pan-orthopox in the CY5 channel and control human RPP30-DNA in the HEX channel
(RUO). Research Use Only. Not for use in Diagnostic Procedures.
Introduction
The kit MPX1-OPV1-RPP30DNA is a multiplexed quantitative polymerase chain reaction (qPCR) test intended for the qualitative detection of nucleic acid from the monkeypox virus.
The MPX1 assay detects the gene for the “B21R Surface glycoprotein cadherin-like domain putative membrane-associated glycoprotein” (MPXVgp182, Protein ID: gb|URK20621.1). The MPX1 assay has been experimentally verified to detect a 500 BP synthetic construct of the Monkeypox virus. The MPX1 assay has also been experimentally validated on extracts of clinical monkeypox positive samples (data not shown). The MPX1 assay is specific for Monkeypox and will not detect most other orthopox viruses. The MPX1 assay has been experimentally validated to NOT detect cowpox, horsepox, racoonpox, or ectromelia viruses (specificity experiments were performed on 500 BP synthetic constructs of the homologous B21R surface glycoprotein genes from each virus). Bioinformatics analysis of the genome homologous B21R surface glycoprotein of variola virus contains many mutations (i.e., 10 mutations in FP and RP regions) compared to monkeypox and thus variola is unlikely to be detected by the MPX1 assay.
The OPV1 assay is intended for detection of nucleic acid from several orthopox viruses and detects the conserved protein coding family called “B4R Schlafen-like (Cop-B2R)” (MPXVgp165 Protein ID: gb|URK20603.1). The assay has been experimentally verified to detect synthetic constructs of: monkeypox, cowpox, horsepox, buffalopox, rabbitpox, ectromelia virus, akhmeta virus, and vaccinia virus. Experimental performance data shown below are for monkeypox only (please contact us if further information about OPV1 detection of other viruses is needed). The OPV1 assay has also been experimentally validated on extracts of clinical monkeypox positive samples (data not shown). The OPV1 assay was experimentally verified NOT to detect raccoonpox. Based upon a bioinformatic analysis of genome sequences of the variola virus (i.e., causative agent of smallpox), the OPV1 assay should NOT detect variola.
This kit is for research use only and should not be used for diagnostic procedures. The performance of this kit was tested using standard synthetic DNA constructs (monkeypox_construct1_DNA and orthopox_construct1_DNA) from Twist Biosciences. The limit of detection (LOD) is below 10 copies/reaction for both MPX1 and OPV1 assays.

Tube 1: 20x Primers/Probe specific for Monkeypox and 20x Primers/Probe specific for human RPP30 gene Intron I.
Contents
A mix of primers/probe targeting the monkeypox virus, orthopox viruses, and human RPP30 DNA Intron I is provided in a tube as a 20´ concentrated working solution. The fluorophore of the probe for monkeypox is FAM™ (Carboxyfluorescein, a trademark of Life Technologies, Inc) and the quencher is BHQ-1™ (Black Hole Quencher, a trademark of Biosearch Technologies, Inc.) The fluorophore of the probe for orthopox is CY5™ (a trademark of Amersham Biosciences Corp) and the quencher is BHQ-2™ (Black Hole Quencher, a trademark of Biosearch Technologies, Inc.) The fluorophore of the probe for human RPP30DNA Intron I is HEX™ (Hexachloro-fluorescein, a trademark of Life Technologies, Inc), and the quencher is BHQ-1™.
A mix of DNA constructs of monkeypox, orthopox and hRPP30_DNA is provided in a separate tube for a triple positive control. The concentration of each DNA construct is 5,000 cps/µL.
Kit Handling and Contamination
The DNA Software kit MPX1-OPV1-RPP30DNA is shipped at ambient temperature, and should be stored at -30 to -15°C. The kit should be kept on ice once thawed.
Any contamination should be avoided by using appropriate personal protective equipment (PPE), powder free gloves, aerosol barrier pipette tips, and a clean hood.
Experimental
Set up your reaction (20 µL) as follows on ice:
| Component | Volume (µL) |
|---|---|
| InhibiTaq mastermix (2x) | 10 |
| MPX1-OPV1-RPP30DNA mix (20x) | 1 |
| Sample | 2 |
| Water | 7 |
Note: The composition of this reaction is calculated based on the user manual of InhibiTaq PLUS qPCR Master Mix, from Empirical Biosciences. In a reaction with the double positive control, 2 µL of the solution from Tube 2 should be added.
A PCR protocol was used in-house for pre-validation on a Bio- Rad CFX96™ Real-Time System, with the following program:
| Step | Thermocycling Protocol: |
|---|---|
| 1 | Incubate @ 95 °C for 2 minutes |
| 2 | Incubate @ 95 °C for 3 seconds |
| 3 | Incubate @ 60 °C for 30 seconds |
| 4 | Plate Read |
| 5 | Go to Step 2, repeat 44x more |
Result Interpretation
After running the qPCR reaction, perform a regression analysis on the data to determine the quantification cycle, Cq. (Cq is preferred over Ct). Each fluorescence channel with a Cq < 38 cycles and final RFU >200 is considered “positive” or “+” in the Table below.
| MPX1 (FAM™) |
OPV1 | RPP30 (HEX™) |
Interpretation and recommendation |
|---|---|---|---|
| – | – | – | The PCR reaction failed. Please repeat the experiment |
| – | – | + | The sample doesn’t contain monkeypox, cowpox, horsepox, buffalopox, rabbitpox, ectromelia virus, akhmeta virus, and vaccinia virus. |
| + | + | – | The sample contains monkeypox DNA. The sample may also contain cowpox, horsepox, buffalopox, rabbitpox, ectromelia virus, akhmeta virus, and vaccinia virus. The sample may not contain human DNA (hRPP30 gene Intron I). |
| + | + | + | The sample contains monkeypox DNA and human DNA (hRPP30 gene Intron I). The sample may also contain cowpox, horsepox, buffalopox, rabbitpox, ectromelia virus, akhmeta virus, and vaccinia virus. |
| – | + | + | The sample does NOT contain monkeypox DNA. The sample contains human DNA (hRPP30 gene Intron I) and at least one of the following orthopox viruses: cowpox, horsepox, buffalopox, rabbitpox, ectromelia virus, akhmeta virus, and/or vaccinia virus. |
Pre-Validation Experiments
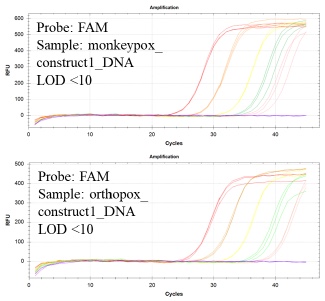
Experiments were performed in triplicate using the experimental procedure given above, but with different samples added to each reaction. The samples used for the validation experiments include water NTC, a water sample spiked with 10000 copies/uL of monkeypox_construct1_DNA, a water sample spiked with 10000 copies/uL of orthopox_construct1_DNA, a water sample spiked with 10000 copies/uL of human RPP30DNA construct, a water sample spiked with 10000 copies/uL of both monkeypox_construct1_DNA and human RPP30DNA construct, a water sample spiked with 10000 copies/uL of both orthopox_construct1_DNA and human RPP30DNA construct, and a water sample spiked with 10000 copies/uL of monkeypox_construct1_DNA, orthopox_construct1_DNA and human RPP30DNA construct. The results of these experiments are shown in Figure 1 below:

Color coding:
10,000 copies
1,000 copies
100 copies
10 copies
1 copy
NTC
Figure 2: Serial dilution experiments show LOD <10 molecules for monkeypox_construct1_DNA and monkeypox_construct1_DNA.
Conclusion: The data in Figure 1 indicate that the primers and probe of MPX1, OPV1, and hRPP30DNA are compatible with each other to detect monkeypox and other orthopox viruses in the matrix of human sample extract.
Limit of detection (LOD) was estimated by performing serial dilution experiments in triplicate (Figure 2). For dilution series only the monkeypox_construct1_DNA or orthopox_construct1_DNA was added. The results show a limit of detection (LOD) <10 copies/reaction in both cases.
Contact Us
For assistance, please contact DNA Software using the link: https://www.dnasoftware.com/contact/
Address:
Michigan Life Science and Innovation Center
46701 Commerce Center Dr
Plymouth, MI 48170
Phone: (734) 222-9080